The quest to discover the basic building blocks of matter has always been an important goal of physicists. Today most people are familiar with the atomic theory, but when it was first fully developed in the early 19th century it was considered a revolutionary conceptual leap. The first complete atomic theory was proposed by John Dalton, and his atomic theory of matter laid the groundwork for modern physics and chemistry.
Background and Development to Dalton’s Atomic Theory
The concept of atoms as fundamental units of matter had been around for centuries, dating back to the ancient Greek philosophers. However, the ideas of the Greeks were purely philosophical and lacked any experimental evidence. They therefore laid mostly dormant for many centuries.
By the start of the 19th century chemistry was undergoing significant changes. The old ideas of alchemy were becoming obsolete and were being replaced by a more systematic approach. Alchemy was the predecessor of modern chemistry, but it was mostly concerned with the transmutation of materials. Beginning in the 17th century, scientists such as Robert Boyle began challenging the idea of alchemy. However, the true chemical revolution came in the late 18th century, particularly with the work of Antonie Lavoisier.
Lavoisier is often referred to as the “father of modern chemistry” and with good reason. His work involved conducting meticulous quantitative experiments which established the Law of Conservation of Mass and refuted the phlogiston theory, the prevailing explanation for combustion and rusting. The Law of Conservation of Mass was a crucial idea that John Dalton used in formulating his atomic theory.
One final idea that was used by Dalton to establish his atomic theory was developed by the French chemist Joseph Proust and is known as the law of definite proportions. Proust demonstrated that chemical compounds always contain the same proportion of elements by mass, implying that elements combine in fixed rations.
The Development of Dalton’s Atomic Theory
John Dalton was an English Quaker who was the first person to put forth a complete atomic theory of matter. Dalton did not come from a wealthy background and his scientific interests developed gradually, after being influenced by a wealthy local Quaker named Elihu Robinson. Robinson spurred Dalton’s interest in meteorology, and in March 1787 he began a meteorological diary where he recorded daily observations until his death in 1844.
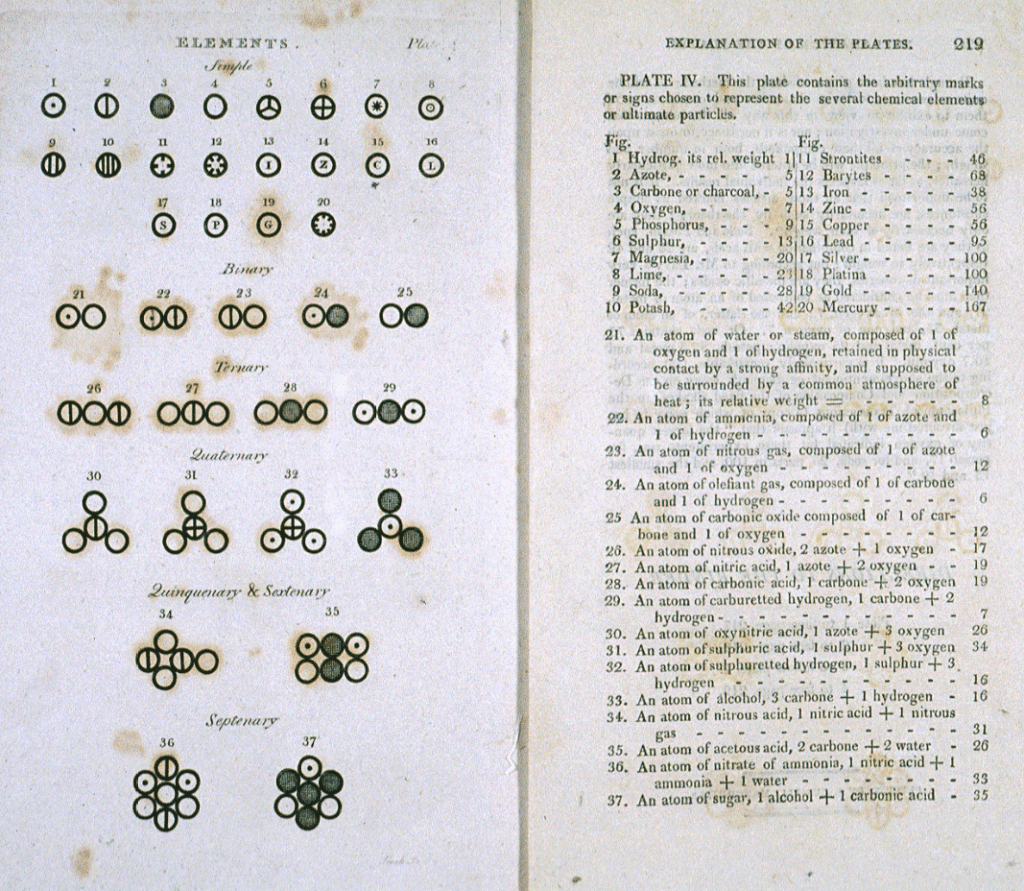
Dalton’s keen interest in meteorology led him to think deeply about the ways gases mix providing the initial steps in his formulation of his atomic theory. He eventually conducted several experiments that were focused on gases, especially what happened when they combined. In 1803, Dalton published his first list of atomic weights for a number of elements base on his experiments and observations. He soon became convinced of a few key principles that became the central principles of his atomic theory. Dalton presented his full ideas in lectures to the Royal Institution in December 1803 and January 1804.
Dalton’s atomic theory consisted of these key principles.
- Elements are composed of atoms: Dalton proposed that all elements are composed of indivisible particles called atoms. These atoms are unique to each element and have certain properties that determine that characteristics of the element.
- Atoms are indestructible and indivisible: Dalton believed that atoms were indestructible and did not consist of any smaller, more fundamental particles.
- Atoms of the same element are identical: Dalton suggested that atoms of the same element are identical in mass, size, and chemical properties.
- Compounds are formed by combinations of atoms: Dalton suggested that compounds are formed by mixing different combinations of atoms of different elements in fixed rations.
- The law of multiple proportions: This law states that when two elements combine to form more than one compound, the masses of one element combine with a fixed mass of the other element in whole number ratios.
In 1808, he published a book titled A New System of Chemical Philosophy, the seminal work that laid the foundation for modern atomic theory. Dalton’s work, however, did not make an immediate impact as many people still found it hard to accept the idea of atoms. Most considered it a useful heuristic device, as a tool to describe how elements behave, but not convinced that atoms are actually real, physical entities. It took another 100 years before the first definitive proof of atoms, when Albert Einstein conducted a series of experiments and in 1905 published his paper on Brownian motion.
Impact and Legacy of Dalton’s Atomic Theory
Dalton’s atomic theory had a profound impact on the development of the emerging science of chemistry, and it laid the groundwork for many further scientific discoveries. It contributed to the development of the periodic table of elements as scientists discovered more elements and their associated properties. It also helped scientists understand chemical reactions better by viewing these reactions as interactions between individual atoms. Although many aspects of Dalton’s atomic theory have been revised and updated based on new discoveries, his core principle of atoms as the basic building blocks of the elements fundamental to modern atomic theory.
Continue reading more about the exciting history of science!

This post took me back to HS and college. I never thought of linking Dalton’s Atomic theory to the periodic table. Thanks for highlighting his contribution to science today.
I must say, this is all new to me. I don’t even remember hearing about it in high school.