The atomic nucleus is the tiny, dense, center of the atom. It’s surprising discovery was announced to the world by the physicist Ernest Rutherford at a meeting of the Manchester Literary and Philosophical Society in March 1911. Two months later he published a scientific paper reporting his findings.
The Rutherford Experiment
Prior to Rutherford’s discovery of the atomic nucleus, the prevailing atomic model was the “plum pudding” model devised by J. J. Thomson, who discovered the electron in 1897. Thomson proposed a model of the atom that consisted of a diffuse cloud of positive charge with the negatively charged electrons scattered within it. However, there was a lack of experimental evidence to support this model, along with other issues such as the problem of atomic stability, and conflictions with observations of atomic behavior.
Rutherford, along with his colleagues at the University of Manchester, set out to further investigate the structure of the atom using alpha particles. Alpha particles are a form of radioactive decay that have a positive charge. Rutherford’s team fired a beam of alpha particles at a thin sheet of gold foil. To create his beam Rutherford placed radium inside a lead box with a small pinhole directed at the sheet of gold foil. The lead box absorbed most of the alpha decay from the radium except for the small beam that escaped through the pinhole. The foil was completely surrounded with a detector that could locate where they alpha particles ended up after they passed through the foil. Rutherford specifically used gold for its malleable properties. Gold can be hammered into incredibly thin layers which was needed so that the alpha beam could pass through it.
Most of the alpha particles passed through the foil as if it was going through empty space. Occasionally a few alpha particles – about 1 in 20,000 – were reflected straight back towards the source. This was a highly unexpected result that could not be explained by the plum pudding model. Rutherford concluded that most of the mass of an atom must be concentrated in a tiny, dense region of the atom which he called the nucleus. He proposed that the atom had a central nucleus where all the positive charge and most of the mass was concentrated, and the negatively charged electrons orbit this nucleus. His model of the atom resembled the planets in the way that they orbit the Sun in the Solar System.
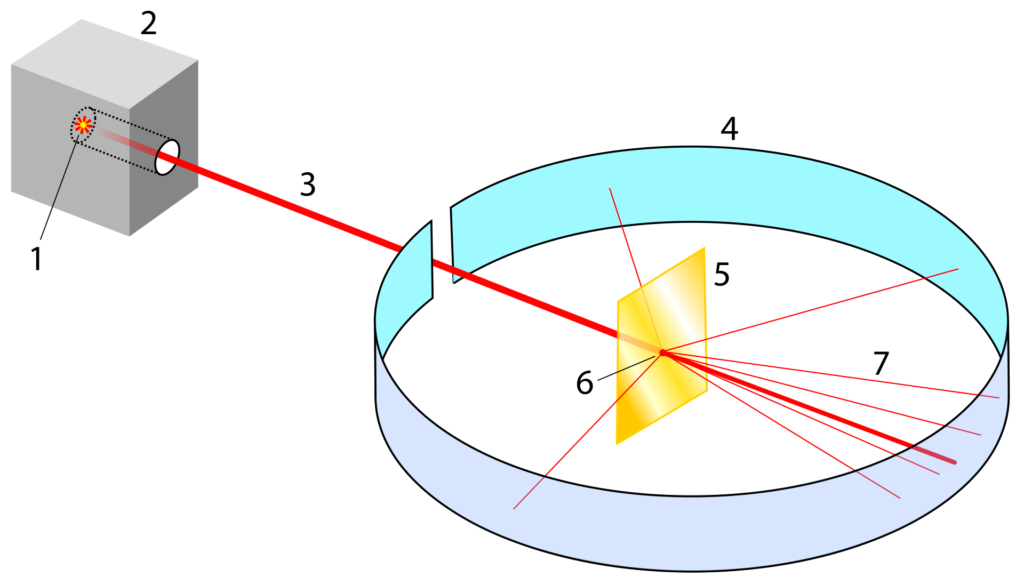
(Credit: Wikimedia Commons)
The Atomic Nucleus and Structure of the Atom
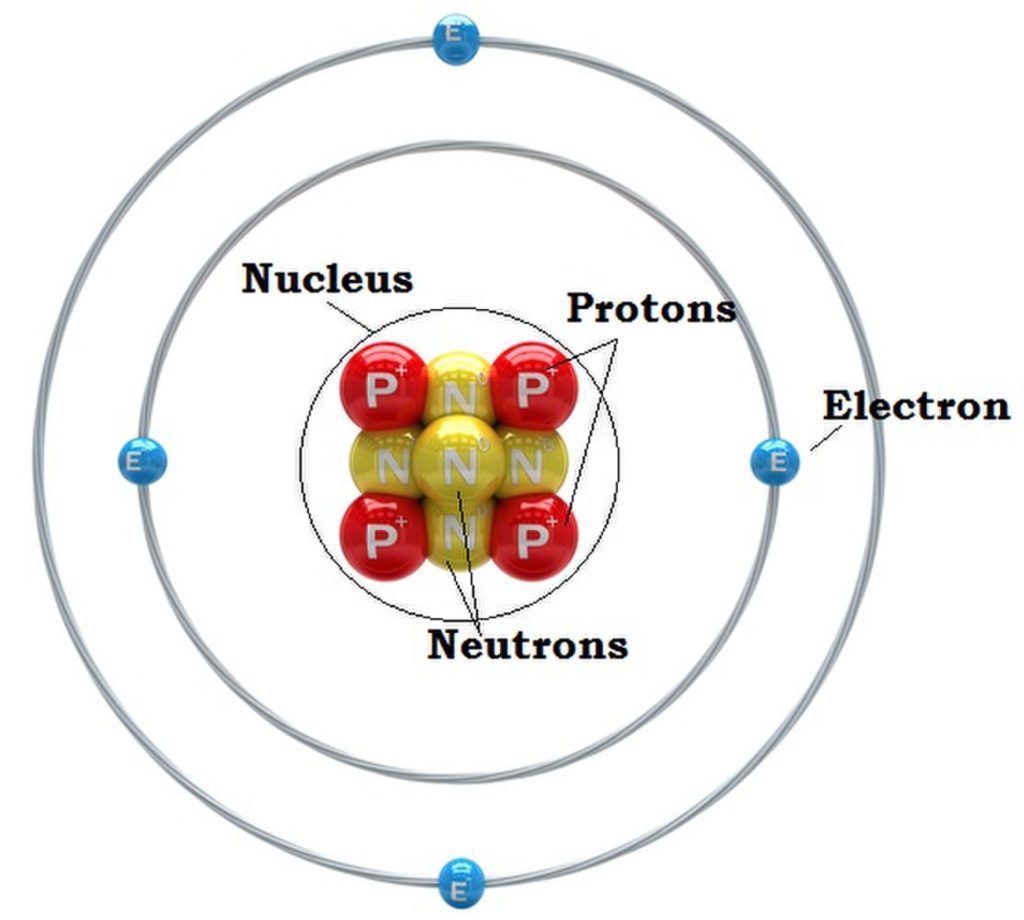
Rutherford’s model of the atom contained the concept of the nucleus, a significant departure from the plum pudding model. It consists of positive electrically charged protons and the slightly heavier electrically neutral neutrons. Although Rutherford failed to discover the neutrons himself he predicted their existence and his student James Chadwick discovered them in 1932. Orbiting the nucleus are the negatively charged electrons.
One of the most striking implications of this model is that atoms are mostly empty space. The atomic nucleus occupies a space of 1/100,000 of the atom, with electrons occupying the vast region around the nucleus. This poses the question: what fills the region between the nucleus and the orbiting electrons? The answer is empty space. This representation challenges our perception of solid matter, and underscores the weird, mysterious, and counter-intuitive nature of the atomic realm.
Although Rutherford’s proposed a model of that atom was a significant improvement over the plum pudding model it still had limitations of its own. Critically, it still could not explain the stability of the electrons’ orbits around the nucleus. According to classical physics, electrons moving in an orbit would radiate energy and soon – within one second – spiral into the nucleus. Obviously, atoms are stable and do not collapse in this way.
The solution to this problem came from Niels Bohr and the quantum mechanical model of the atom. Bohr introduced the concept of quantized orbits for electrons, where electrons could only exist at certain discrete energy levels without radiating energy. According to Bohr’s theory, electrons moving between orbits actually disappear from one orbit and reappear in the new orbit, without traveling in the space between. This resolved much of the stability issues despite reinforcing the confusing and unusual ways that atoms behave at the subatomic scale.
The discovery of the atomic nucleus had profound implications on atomic physics and led to the development of an entirely new field of research, nuclear physics. It paved the way for the harnessing of nuclear energy, a technology that has the potential to alter the course of human civilization.
Continue reading more about the exciting history of science!
Very imformative! My husband loves stuff like this and finds it super interesting
Thank you for sharing! Great information, interesting and easy to follow.
SO much information! I just taught my six year old about Atoms, molecules and more this past weekend!
Very informative write-up. This is something I learned during my high school and University time. Great to read it again just to refresh.
What an interesting and informative read. I love learning new things, and this was easy to follow for someone like me who isn’t in a science-related field.
this is an hubby for my brother but i will say it was very informative
What an informative post… I like that it is written so that even someone like me who is not into science can understand it!
This absolutely fascinates me! It’s insane what technology and science is able to uncover these days! And I can only imagine the new discoveries we will find in the near future!
oh wow!! This is really insane and super cool to learn about! Wonder what’s next?!
I remember learning about this in school. I found it so fascinating but have lost a lot of that knowledge. This is a nice tutorial!
Very fascinating! Technology amazes me every day and i’m both curious and afraid for the future
Great way to refresh my memory. It was written in a way that you can easily understand.
I love the way it is written, easy to understand, very informative, such a nice article.. 😊
It always fascinates me to learn about the different charges in the nucleus. I remember balancing the charges as a kid.
Wow, thanks for a really good explanation of the atom and the atomic nucleus. How it was tested and by whom is so informative.
Cool, I definitely learned something reading this article
Thanks for the amaizing explanation. ❤
I already read this fact years ago from a book, reading it here with those kinds of fonts makes it less boring and entertaining to read.. Thanks for sharing..
The information shared in this blog post s absolutely fascinating about atoms.
Keep writing more and fascinating me.
I seriously had no idea about all these. Very informative!
it is fascinating to know the outcome of this kind of experiment. It is very informative, recalling the science class I had during my school days 🙂
Very valuable article… Visit http://www.kyakaisehai.com for interesting fact and biography related Post