In 1913 Niels Bohr proposed a model of the atom based on quantum mechanic physics which helped solve problems of previous atomic models that were based on classical physics. His proposal came to be know as the Bohr model of the atom.
Earlier Models of Atomic Structure
Prior to Bohr’s model of the atom there were various competing models being used. In 1897 J.J. Thomson discovered the electron through his experiments with cathode ray tubes, setting the stage for the development of his plum pudding model. In this model electrons were embedded in a positively charged sphere, akin to plums scattered in a pudding.
Shortly afterwords, Ernest Rutherford announced the surprising discovery of the atomic nucleus which instantaneously and radically transformed our understanding of the structure of the atom. The discovery of the nucleus paved the way for Rutherford’s nuclear model of the atom, placing the nucleus in the center with electrons orbiting around it, akin to planets orbiting the Sun in our Solar System. Unfortunately, both of these models had shortcomings because they were entirely based on classical physics.
The most pressing issue was the stability of the atom. According to electromagnetic theory, whenever an electron is accelerated around the nucleus of an atom it should emit radiation resulting in a continuous loss of energy. This loss of energy would cause the electron to slow down and spiral into the nucleus almost instantly. Clearly this does not happen in the real world as atoms are stable. To solve for this problem Bohr used the emerging quantum physics.
Bohr’s Model of the Atom
Bohr’s model resolved this problem by showing the electrons in orbit were consistent with Max Planck’s quantum theory of radiation. At the turn of the 20th century Max Planck introduced the revolutionary concept of quantized energy to explain the spectrum of black-body radiation. His key insight was that energy is emitted or absorbed by matter in discrete units, or “quanta,” rather than in a continuous manner as predicted by classical physics. This insight laid the groundwork for Bohr’s work on the structure of the atom.
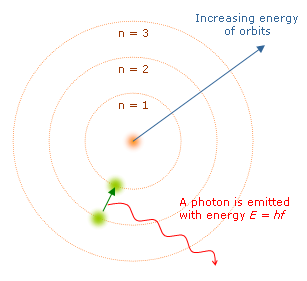
In Bohr’s model of the atom electrons would only be able to occupy certain orbits with a specific amount of energy, which he referred to as energy shells or energy levels. They emit or absorb radiation only when electrons abruptly jump between different orbits. Using Plank’s constant, the frequency of photons, and some information about the electrons mass and charge, Bohr was able to obtain an accurate mathematical formula for the hydrogen atom.
This was huge improvement on previous models because it incorporated the new quantum physics, however there still were a few problems associated with Bohr’s model. It was not a particularly useful description for atoms other than hydrogen and it failed to account for the Zeeman Effect in hydrogen. It was eventually refined and superseded by quantum theory that was consistent the work of Werner Hiesenberg, Erwin Schrodinger, Max Born, and many others.
Impacts of Bohr’s Model of the Atom
Bohr’s model of the atom, with its quantized energy states, was nothing short of revolutionary. It implied several significant impacts on the understanding of atomic structure and on the development of quantum mechanics. Some of the key impacts of Bohr’s model include:
(Credit: www.webbtelescope.org)
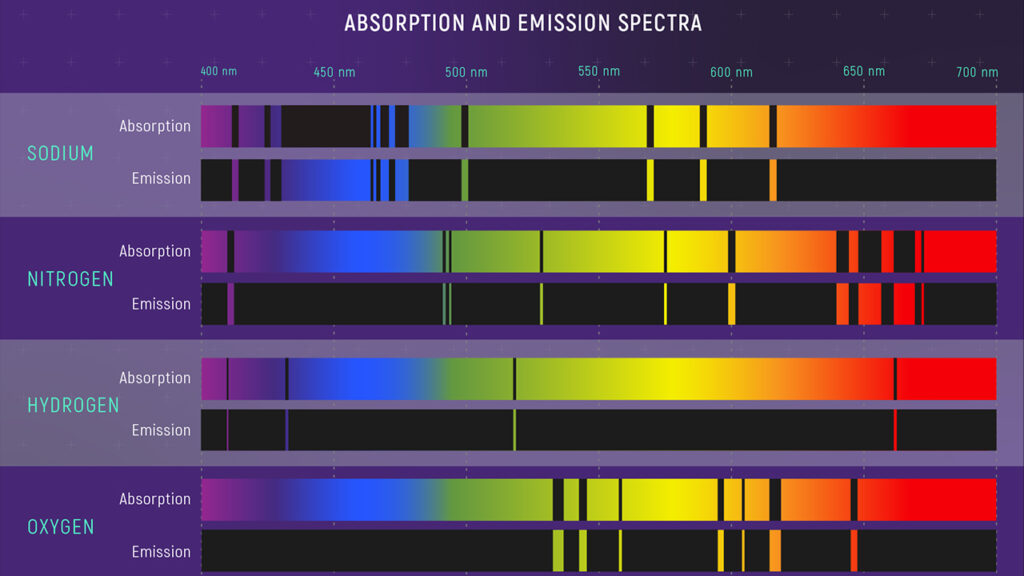
- Explanation of atomic spectra: Bohr’s model was successful in explaining the discrete line spectra observed in the emission and absorption of light by atoms. It provided a theoretical framework for understanding the spectral lines of different elements.
- Development of quantum theory: Bohr’s model was crucial in the early development of quantum theory. It provided one of the first examples of the application of quantum principles to the behavior of electrons in atoms.
- Influence on atomic theory: Bohr’s model spurred further research and inspired subsequent scientists, including Werner Heisenberg, Erwin Schrodinger, and Max Born. These scientists went on the develop more sophisticated quantum mechanical models.
- Practical technological applications: Bohr’s model has helped in the development of technologies such as lasers, semiconductors, and nuclear energy. These technologies depend on an understanding of atomic behavior.
Overall, Bohr’s model of the atom had a significant impact on physics as a whole. Its development can be marked as a transition in time between classical physics and quantum mechanics. While his model has since been superseded by a more comprehensive quantum mechanical model, its significance as a foundational role in the development of atomic theory and quantum mechanics remains important.
Continue reading more about the exciting history of science.