The neutron was discovered by the British physicist Sir James Chadwick in 1932, marking a pivotal moment in the understanding of atomic structure. It was, in a way, the culmination of a series of scientific investigations of the subatomic particles of the atom that spanned several decades. The identification of the neutron provided answers to questions about the mass of the atom, ultimately leading to important developments in nuclear physics.
Developments that Led to the Discovery of the Neutron
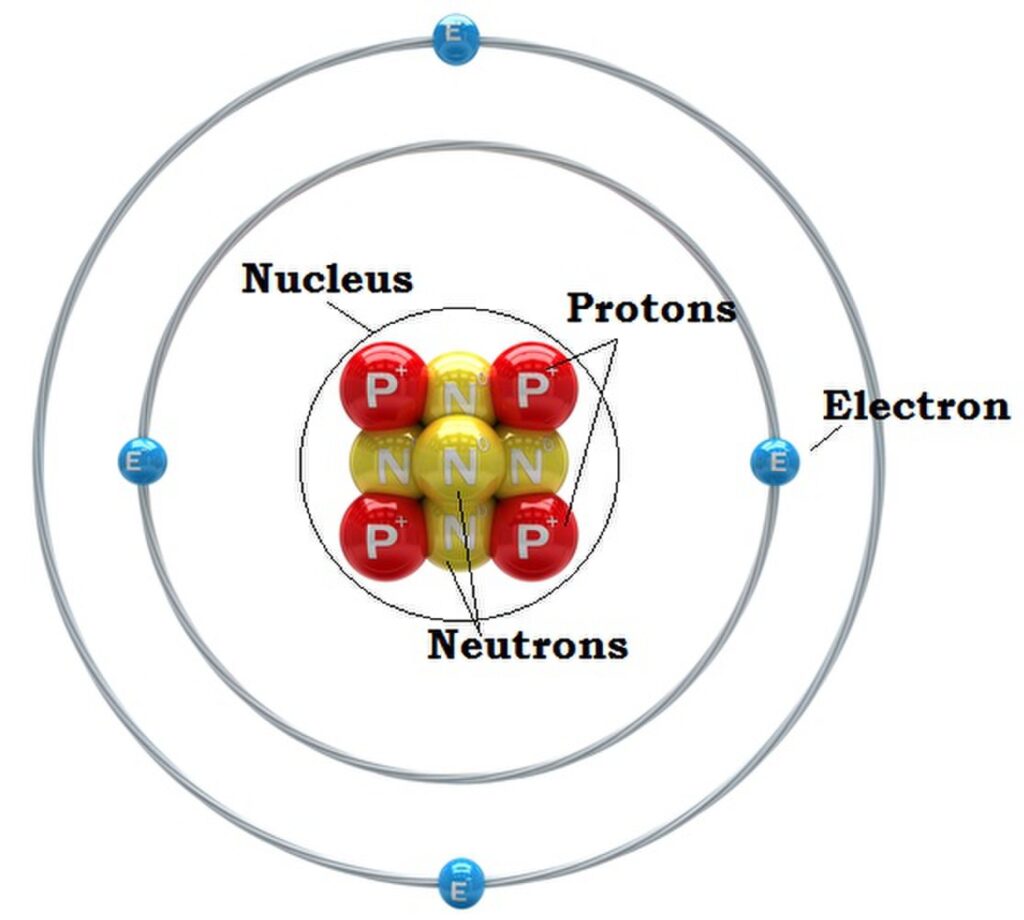
In 1897, J. J. Thompson discovered the electron, a subatomic particle with a negative electrical charge. This discovery provided the evidence that atoms were composed of smaller particles. Two decades later, Ernest Rutherford discovered the proton, a subatomic particle with a positive electrical charge. Rutherford proposed a model of the atom with a dense, positively charged nucleus at its center, orbited by negatively charged electrons. However, this model presented a problem. The positively charged protons in the nucleus should all repel each other, causing the nucleus to burst apart. Yet, this did not happen as the nucleus is obviously stable, and the reasons for this stability were not understood at the time. The existence of a neutral particle was postulated by Rutherford as early as 1920.
The Discovery of the Neutron
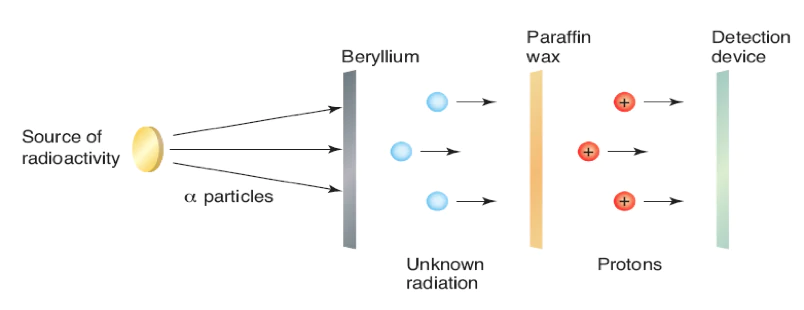
This discovery of the neutron was the culmination of a series of successive experiments, worked out by several scientists in the late 1920s and early 1930s. In Germany, Walter Bothe found that beryllium exposed to alpha particles produced a new form of radiation. Bothe attempted to explain this sradiation in terms of gamma rays, because it was not deflected by either electric or magnetic fields. All known particles at the time (electrons and protons) contained a charge.
Taking this information a step further, the French husband-and-wife team of Irene Joliot-Curie (the daughter of Pierre and Marie Curie) and Frederic Joliot reported results from an experiment in January 1932 that led to to neutrons discovery. In the same vein as Bothe, their experiment involved the bombardment beryllium by alpha particles. They noticed that the radiation could eject protons from hydrogen-rich substances such as paraffin. This was puzzling because gamma rays should not have enough energy to be able to knock out protons in this manner. In other words if this unknown radiation was indeed gamma rays then the law of conservation of energy was being violated.
Back at the Cavendish Laboratory in Cambridge, James Chadwick, a college or Rutherford, quickly became interested in these results. Chadwick and Rutherford had been working on and off over the past decade in identifying the missing neutral particle suspected to be in the atomic nucleus. This background allowed Chadwick to move quickly. He also conducted a series of experiments where he bombarded light elements, such as beryllium, with alpha particles. He noticed the same radiation being emitted which was not deflected by electric or magnetic fields. However he interpreted the results differently than the others who conducted similar experiments. His observations led him to correctly conclude that the radiation was composed of uncharged particles. He used the laws of conservation of momentum and conservation of energy to calculate that the neutron has a mass similar to that of a proton. He presented this as evidence of a new subatomic particle, which he detailed in a paper published in 1932 and named the neutron. For his work he was awarded the Noble Prize in Physics in 1935.
Impact of the Neutron’s Discovery
The discovery of the neutron was a key piece of the puzzle that allowed scientists to understand the binding energy of the atomic nucleus. It had enormous impacts in both applied and theoretical physics.
The discovery of the neutron explained the missing mass in atomic nuclei. This in turn helped to explain the existence of isotopes – variants of elements with the same number of protons but different atomic weights – and therefore different numbers of neutrons in the atomic nuclei. It also advanced the understanding of radioactive decay processes.
The most important impact of the discovery of the neutron was in nuclear physics. The neutron – a particle without an electric charge – was the crucial component in the development and study of nuclear fission, which occurred in 1938 by Otto Hanh and Lise Meitner. The development of nuclear fission was quickly applied the development of nuclear energy and weapons. The neutron plays the key role in the chain reactions that occur in both nuclear reactors and atomic bombs. It is probably fitting then, that James Chadwick was placed as head of the British team that work on the Manhattan Project that produced the world’s first atomic bomb.
Beyond nuclear fission, the discovery of the nucleus aided in our understanding in the nuclear processes that power the stars through the process of nuclear fusion. The study of this process had advanced our understanding on the origins and evolution of the elements.
Continue reading more about the exciting history of science!