The battery has revolutionized the way we live, producing a reliable and portable power source for a wide range of products. The concept of batteries has a rich history that spans centuries, possibly even millennia. As far back as the 1st century AD, civilizations may have been experimenting with battery concepts, with artifacts discovered around Baghdad that resemble battery-like devices. However, the true purpose of these devices remains controversial.
Alessandro Volta and the Birth of the Battery
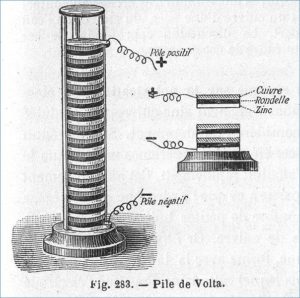
At the turn of the 19th century electricity was becoming an increasing topic of study. People were finding various ways to produce or store electric current but there was as of yet no way to produce a continuous flow of electricity.
In 1799 the Italian physicist Alessandro Volta solved this problem and first reported his findings in a letter to the Royal Society in 1800. Called a Voltaic pile, Volta stacked zinc, copper, and brine-soaked paper in layer after later, sometimes referred to as a voltaic cell. Adding a wire to both ends produced an electric current, with additional layers creating a stronger current. These additional layers stacked on top of each other created the voltaic pile. Volta realized that somehow the piles of metal disks were producing the current, an effect called an electromotive force.
In 1810 Humphry Davy showed that it was the chemical reaction between the two metals (electrode) and the liquid solution (electrolyte). Various difference metals and solutions can be used all to create an electromotive force.
The voltaic pile marked a significant milestone in the history of electricity. It was the first true electric battery, setting the stage for the development of modern batteries.
Unleashing the Power of Chemical Energy
A battery is then a chemical means of generating electricity. It works through a “redox” reaction, which is the process of reduction (one substance gaining electrons) and the process of oxidation (one substance losing electrons) occurring simultaneously. To perform the redox reaction most batteries consist of two electrodes – an anode (negative electrode) and a cathode (positive electrode) – separated by an electrolyte, which is a conductive material. The electrodes are typically made of two different materials, and the electrolyte allows ions to flow between them while preventing contact. When the battery is connected to an external circuit, the redox chemical reaction occurs within the battery, producing the flow of electrons from the anode to the cathode, creating an electrical current that can be used to power devices.
Volta’s battery is called a wet battery. While it did produce a controlled, continuous flow of electric current it was not very practical. There were significant limitations with its capacity, size, and portability. The battery was a large and cumbersome device and had a low energy density compared to modern batteries. What it was however, was a groundbreaking invention that paved the way for further development of battery technology. It was not until a dry battery was invented, which replaced the liquid electrolyte with a paste, that batteries portable and practical.
The Rise of Practical Batteries
The battery has undergone significant changes in the past two centuries since the invention of the voltaic cell. Here are a few notable improvements:
- Daniell Cell: In 1836, the British scientist John Frederic Deniell invented a new cell using copper sulfate and zinc sulfate, separated by a porous pot, to create a longer-lasting source of power.
- Lead-Acid Batteries: In 1859 the French physicist Gaston Plante invented the first rechargeable battery.
- Leclanche Cell: In 1866, the French engineer Georges Leclanche patented a new kind of battery – a predecessor to the modern dry cell battery. It used a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride.
- Nickel-Cadmium Batteries: In 1899 the Swedish inventory Waldemar Jungner invented the first alkaline battery.
- Lithium-ion Batteries: Lithium is the metal with the greatest electrochemical potential. These batteries did not come to market until the 1970s and offer the highest densities and can hold its charge for the longest period of any battery.
(Credit: aec.org)
Peering into the future batteries will increasingly play a role in society. Emerging technologies such as artificial intelligence (AI), renewable energy storage, advanced portable devices, and electric vehicles (EVs) will need powerful batteries to conveniently function. As a result many of these industries are investing heavily in battery technology. Improved battery technology will enable the widespread adoption of many of these technologies, removing their limitations and making the more appealing to consumers.
Continue reading more about the exciting history of science!