The Carnot cycle is the ideal cycle of operations that provides the maximum theoretical efficiency for any engine that utilizes heat. It was first proposed by the French physicist Sadi Carnot in 1824 prior to its expansion by other scientists.
Reflections on the Motive Power of Fire
Nicolas Leonard Sadi Carnot is often referred to as the “father of thermodynamics”. In 1824, at the age of 27, he published his only book titled Reflections on the Motive Power of Fire in which he introduced the concept of the Carnot cycle while laying down the foundations for a new discipline – thermodynamics. By this time the steam engines industrial and economic importance had been established, although little scientific work had been done by studying them. Carnot’s book was one of the first scientific studies of steam engines.
Carnot explore some key ideas in his book. He recognized heat as a form of energy that can be converted from one state to another. This concept eventually led to the first law of thermodynamics, which states that energy is conserved in any thermodynamic process. He introduced the notion of an idealized heat engine, known as the Carnot engine, as a theoretical construct to study the maximum efficiency of heat conversion. This engine could be served as a reference point against which all other real work engines could be compared. Additionally, he noted that the efficiency of a heat engine depends of the temperature difference between the source and the sink, and that maximum efficiency is only achieved when it operates in a recoverable manner.
To understand the principles of an ideal heat engine, Carnot devised the Carnot cycle which is a series of four reversible processes. It is a theoretical cycle that provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work. The Carnot cycle heat engine is not a practical engine that can be made because its processes are reversible, which violate the second law of thermodynamics.
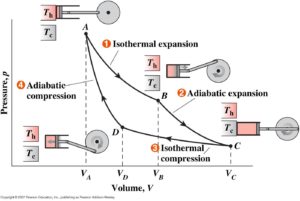
The Four Stages of the Carnot Cycle
The Carnot cycle consists of several operations. First, the engine absorbs energy in the form of heat from a reservoir. Work is done by the heat which causes an expansion. Next is compression where the heat is given out. The net result is that heat is taken from a hot source to a cooler one, while some of the heat does work. The Carnot cycle establishes the highest possible efficiency – measured by dividing the work that is done by the energy absorbed from the reservoir – for any heat engine. In the real work efficiency of an engine is never as high as that predicted by the Carnot cycle due to factors such as friction.
Here are the four stages of the Carnot cycle.
- Isothermal expansion – gas expands on its surroundings as heat is transferred from hot reservoir at a constant temperature; work being done = heat supplied.
- Adiabatic expansion – gas continues to expand on its surroundings with no heat exchange, causing temperature to cool; work being done > heat supplied.
- Isothermal compression – surroundings compress gas as heat is transferred to a low temperature reservoir at a constant temperature.
- Adiabatic compression – surroundings compress gas with no heat exchange, resetting the system to its original state (before stage 1).
The Importance of the Carnot Cycle
Sadi Carnot’s work brought about a revolution in our understanding of the underlying principles of heat transfer and energy conversion. Most directly, it set the stage for subsequent advancements in the field of thermodynamics. Work on the Carnot cycle heat engine led to the fundamental thermodynamic principle of entropy, a fundamental concept related the the second law of thermodynamics. Rudolf Clausius introduced the term entropy in the mid-19th century to explain the irreversible nature of energy transfer and transformations in physical systems. Clausius observed that heat naturally flows from a higher temperature region to a lower temperature region and that the process is irreversible. He recognized the importance of Carnot’s work with heat and sought to put in on a more rigorous, mathematical footing.
Another Rudolf was to later be inspired by the Carnot cycle when he was developing a more efficient engine. In 1982 Rudolf Diesel submitted design patents for an internal combustion engine. He knew gasoline engines were very inefficient, wasting around 90% of its heat in the process. He sought to make an engine closer to the theoretical framework of the Carnot cycle engine.
Continue reading more about the exciting history of science!