The periodic table of the elements is a cornerstone of modern chemistry and an iconic visual representation almost all school children are familiar with. Its origins trace back to 1869, when Russian chemist Dmitri Mendeleev, the inventor of the periodic table in 1869, published his groundbreaking system. Aside from a few minor changes we still use Mendeleev’s system of organization for our modern periodic table.
The Development of the Periodic Table of Elements
By the middle of the 19th century scientists had discovered dozens of elements, but there was no way or organizing them. By 1869 there had been a total of 63 elements discovered and isolated. As the number of known elements was increasing, several scientists began to notice relationships among some of elements and patterns in how they combined with each other. Scientists had been trying to develop a classification system of elements for decades for the known elements for decades but no agree upon system had been reached. For one example, an English scientist named John Newlands proposed the Law of Octaves in 1865. He noticed that every eighth element shared similar characteristics when arranged by atomic weight, much like the notes in a musical octave. In music, an octave consists of eight notes, with every eighth notes a repetition of the previous note just at a higher pitch.
There were however limitations to his law. It has limited applicability, working reasonably well for the lighter elements but breaking down for heavier elements. It also had limited predictive power as it made no room for undiscovered elements. For these and other reasons it was not accepted by all scientists of the time, although it did have a role in the evolution of the periodic table by emphasizing the importance of looking for patterns in the elements of nature.
The Inventor of the Periodic Table in 1869: A Visionary Approach
Enter Dmitri Mendeleev, a chemistry professor at the University of St. Petersburg and the true inventor of the periodic table. In 1861, at age 27, he earned his doctorate after studying abroad in Heidelberg, where he worked with leading chemists like Robert Bunsen, sharpening his analytical skills. A few years later, in 1867, he began working on publishing a chemistry textbook titled Principles of Chemistry. It was his research for this textbook that led him develop this relationship between the chemical properties of the elements to their atomic weights.
Mendeleev’s journey to becoming the inventor of the periodic table in 1869 was not without its quirks and obstacles. Legend has it that Mendeleev conceived the periodic table’s structure in a dream after struggling with the problem for days, arranging element cards on his desk like a game of solitaire.
In any case, his key breakthrough came when he organized his table in order of increasing atomic weight. He placed elements with similar properties underneath each other. In a few instances where it made sense, he swapped some elements out of order of increasing atomic weight to better line up the chemical properties. In doing this he inadvertently set up his table by increasing atomic number rather than atomic weight. By the time his table was finished he had discovered what is now called the Periodic Law, which means that the physical and chemical properties of the elements repeat in a periodic manner.
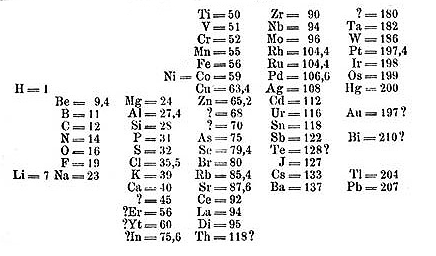
Mendeleev’s true genius came in the fact that he left spaces on his table, correctly predicting the existence of elements that had yet been discovered. He even predicted the properties of these missing elements based on their position in his table. For instance, he correctly predicted the existence of elements that would later be known as gallium, scandium, and germanium. Although his table was initially met with skepticism, when these elements were eventually discovered and their properties closely matched Mendeleev’s predictions it provided a strong validation of his table, quickly leading to its acceptance.
The Modern Periodic Table
Mendeleev’s table has continued to evolve as new elements were discovered and the understanding of the atomic structure increased. It is a product of the collaborative efforts of many scientists involving a few important changes and additions.
Most notably, thanks to the work of Henry Moseley, we now organize the table by atomic number, which is the total number of protons in the nucleus. This change happened as a result of the discovery of isotopes and lead to the realization that atomic number is the fundamental basis for the organization of the elements. In addition to that change the modern periodic table now includes over 100 elements, up from the 63 known to Mendeleev when his first table was published.
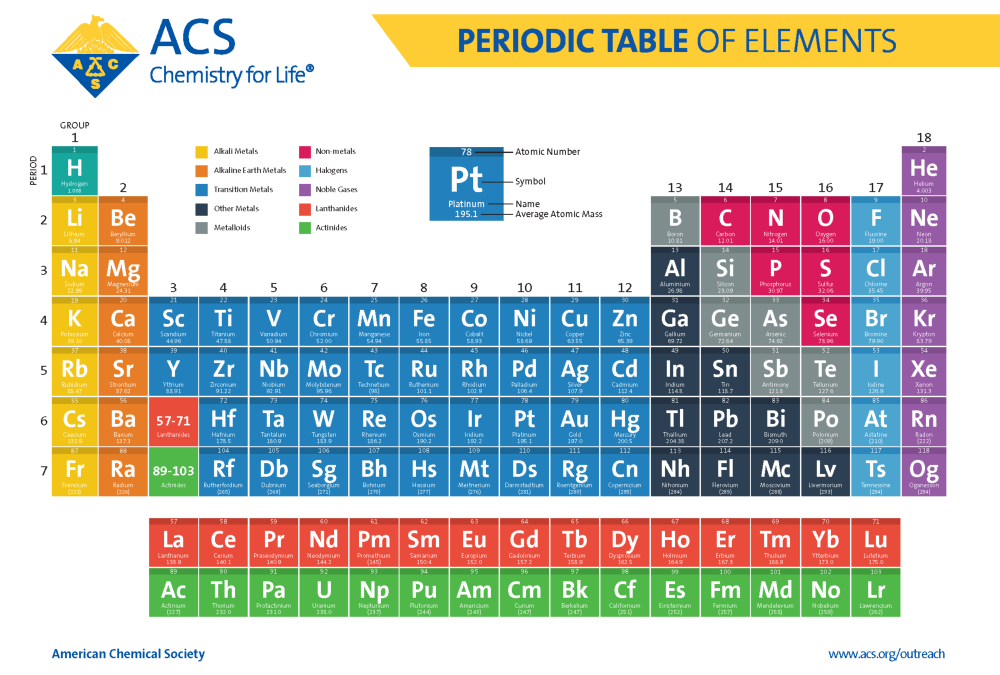
(Credit: American Chemical Society)
The are a few other changes and additions to make note of.
- Noble gases: the original table did not include the specific group of noble gases as these elements were not yet discovered.
- Electron structure: the modern periodic table is often presented with electron configurations for each element.
- Improved measurements: significant advances in technology have allowed for more precise measurements of atomic weight and structure.
- Filling of d- and f- blocks: these blocks were not fully understood during Mendeleev’s time and have been refined to reflect their electronic structures and chemical properties.
- More comprehensive periodic trends: the modern periodic table provides more information about periodic trends such as atomic radius, ionization energy, and electron affinity.
The Importance of the Periodic Table
The periodic table is an indispensable tool for chemists and educators worldwide. Its organization captures the complexity of the natural world in a simple framework that easily shows the relationships, properties, and reactivity between the chemical elements.
The periodic table also provides a wealth of information about the chemical elements. It contains information about the atomic structure and weight, electron configuration, valence electrons, and chemical reactivity. All of this information provides insights into studying the elements and for manipulating matter at the molecular level. One area of study where this information is particularly useful is in the field of material science. By understanding the periodic trends, scientists have been able to design and engineer new materials with specific characteristics for a wide range of applications such as in electronics, energy production, agriculture, and medicine.
The periodic table of the elements was a triumph in scientific reasoning due to its predictive power. It stands as one of the most celebrated foresights in the history of science, serving as a prime example of the power of science to uncover hidden order in the universe.
Continue reading more about the exciting history of science!
